ELECTRONIC CONFIGURATION: Arrangement of Electrons into shells for an Atom (E.g Electronic Configuration of Carbon is 2 . 4 )
s
ELECTRONIC CONFIGURATION AND POSITION IN PERIODIC TABLE
s- Number of notations in electronic configuration will show the number of shells of Electrons the Atom has, showing the Period
- Last Notation shows the number ofouter Electrons the Atom has, showing the Group
EXAMPLE: Electronic Configuration of Chlorine:
PERIOD: Red numbers at the Bottom Show the number of notations which is 3, indicating that Chlorine atom has 3 shells of Electrons
GROUP: Green box highlights the last notation which is 7, indicating that Chlorine atom has 7 outer Electrons
ON THE PERIODIC TABLE: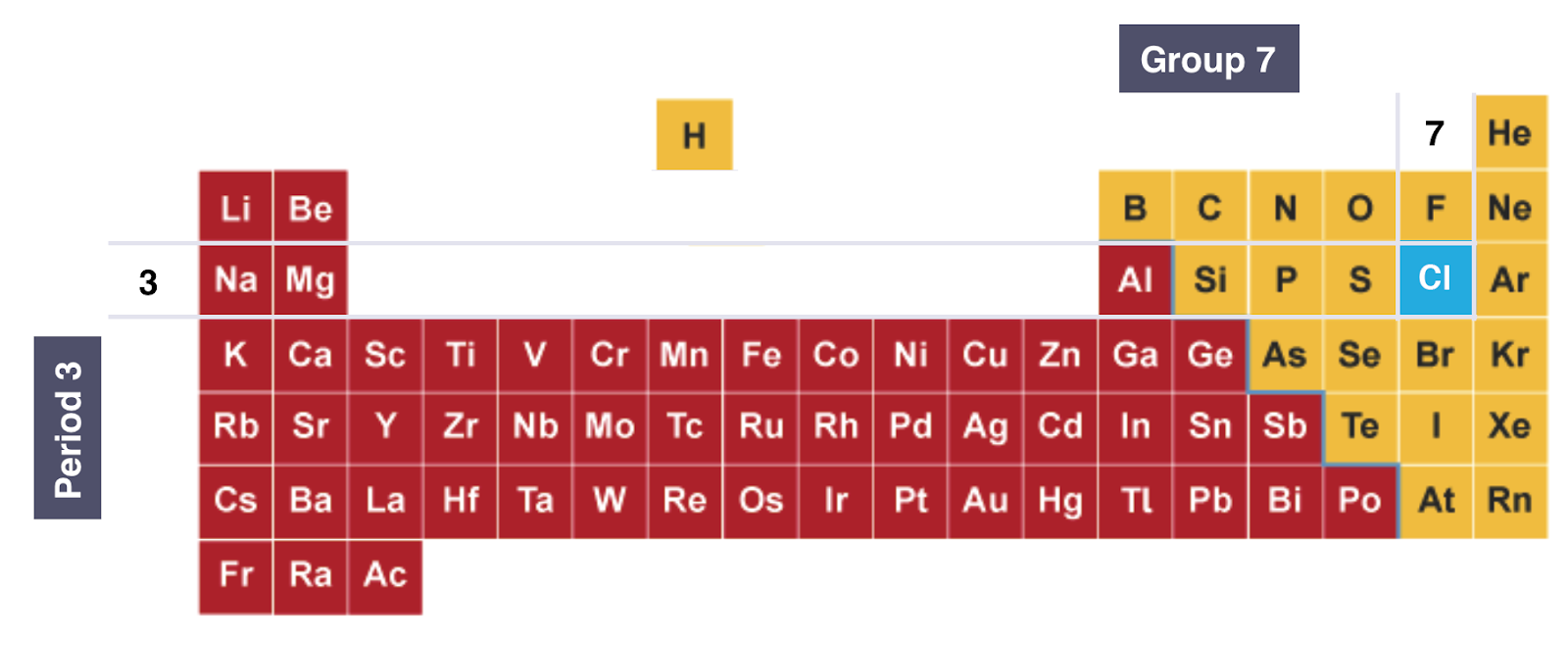
Diagram showing the position of Chlorine on the Periodic Table
Its is a informative post to us really I read & like this blog post.
ReplyDeleteAqueous Products California
Your post is providing some really good information. I liked its essence and enjoyed reading it. Keep sharing such important posts about this blog and its much more helpful for us . crescent ctk170cmp2
ReplyDeleteThis blog is really helpful regarding all educational knowledge I earned. It covered a great area of subject which can assist a lot of needy people. Everything mentioned here is clear and very useful.
ReplyDeleteคลิปบอล
thanks helps alot with my test tomorrow
ReplyDeletePeriodic Table Elements Get all information periodic table elements,Atomic number and Atomic mass number.
ReplyDeleteSometime few educational blogs become very helpful while getting relevant and new information related to your targeted area. As I found this blog and appreciate the information delivered to my database.
ReplyDeleteรองพื้น Revlon
I welcome all the suggestion mentioned in this blog related to new learning skills. It is definitely going to help me to adopt new exited way of learning. I think, others will also feel helpful this blog for their needs.FORTIAP 221C
ReplyDeleteHi i really like your blog and i find it very helpful as im my gcses are approaching but is there no physics? please help
ReplyDeletePleasant to peruse your article! I am anticipating sharing your enterprises and encounters.Black Friday Store Deals
ReplyDeleteI recently came across your blog and have been reading along. I thought I would leave my first comment. I don't know what to say except that I have enjoyed reading. Nice blog. I will keep visiting this blog very often. 구글 정보이용료 현금화
ReplyDeleteIt is a great website.. The Design looks very good.. Keep working like that!. irf1010e equivalent
ReplyDeleteThank you very much for this useful article. I like it.Tables
ReplyDeleteI really like reading through a post that can make people think. Also, many thanks for permitting me to comment! Dofollw Backlinks
ReplyDeleteThanks for sharing us. น้ำยาบุหรี่ไฟฟ้า pod
ReplyDeleteI definitely enjoying every little bit of it. It is a great website and nice share. I want to thank you. Good job! You guys do a great blog, and have some great contents. Keep up the good work. huawei screen repairs
ReplyDeleteThe post is written in very a good manner and it contains many useful information for me. easybom
ReplyDeleteI impressed by the quality of information on this website. There are a lot of good resources here. I am sure I will visit this place again soon.
ReplyDeleteCompany Logo Tablecloth
Reliable snack time partner. buy vending machine
ReplyDelete