ELECTRONIC CONFIGURATION: Arrangement of Electrons into shells for an Atom (E.g Electronic Configuration of Carbon is 2 . 4 )
s
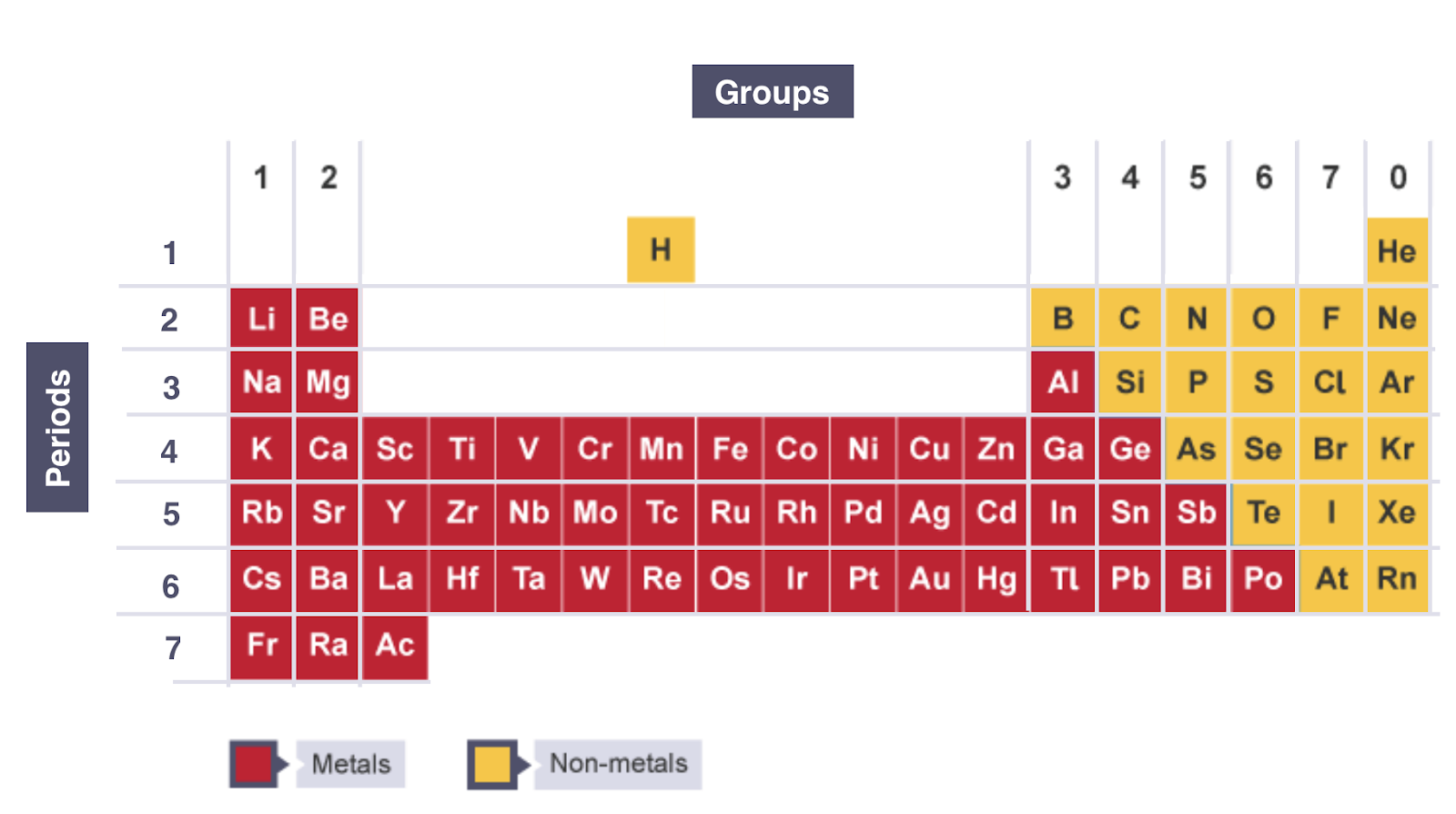
ELECTRONIC CONFIGURATION AND POSITION IN PERIODIC TABLE
s
Number of notations in electronic configuration will show the number of shells of Electrons the Atom has, showing the Period
Last Notation shows the number ofouter Electrons the Atom has, showing the Group
EXAMPLE: Electronic Configuration of Chlorine:
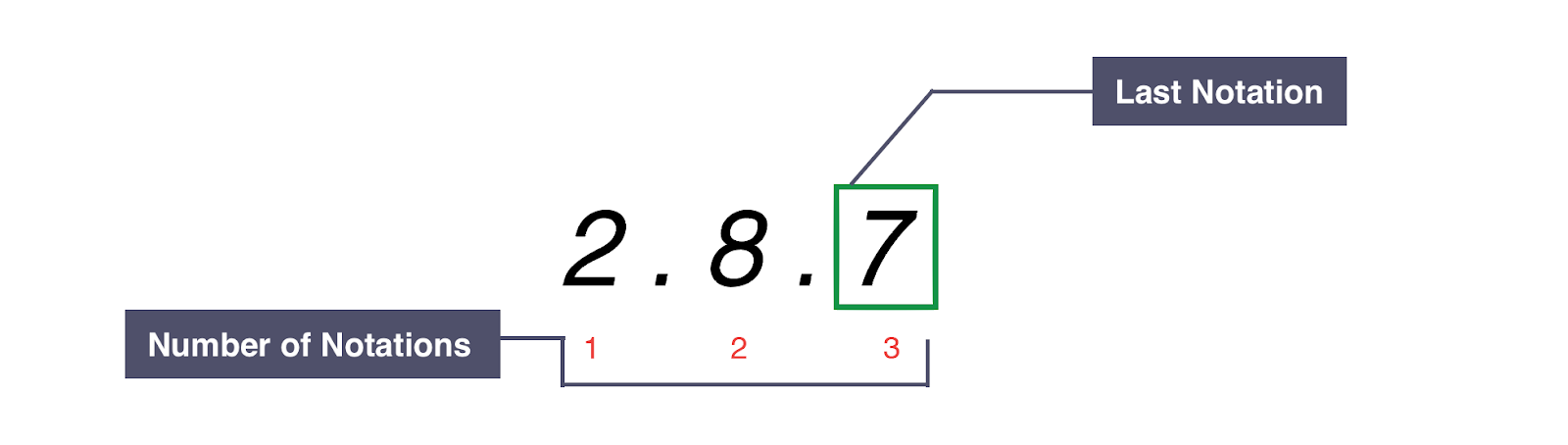
PERIOD: Red numbers at the Bottom Show the number of notations which is 3, indicating that Chlorine atom has 3 shells of Electrons
GROUP: Green box highlights the last notation which is 7, indicating that Chlorine atom has 7 outer Electrons
ON THE PERIODIC TABLE: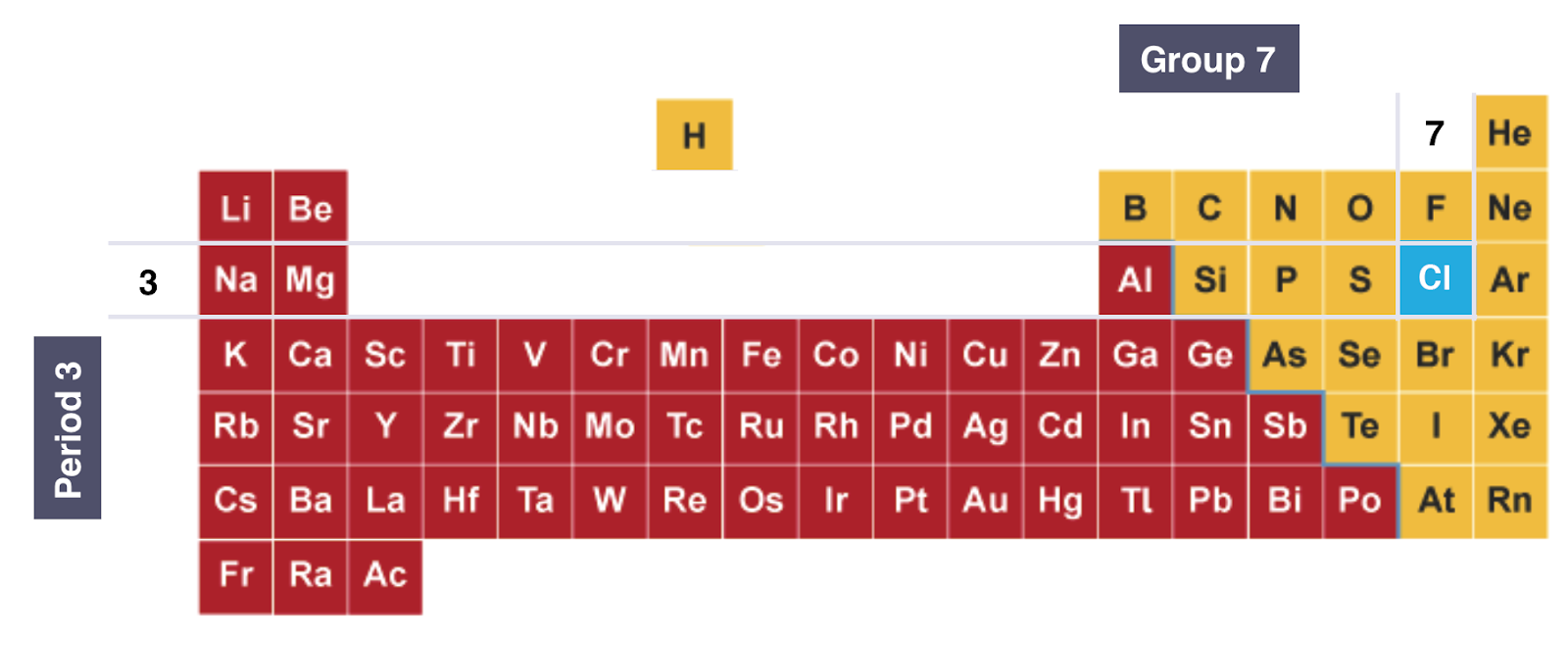
Diagram showing the position of Chlorine on the Periodic Table